Abstract
Background: Relapse is the major cause of treatment failure in acute myeloid leukemia (AML). Patients with AML who are ineligible for allogeneic stem cell transplantation (SCT) have limited options to delay or prevent relapse once they have completed their initial therapy. Oral azacitidine (CC-486) has been shown to improve relapse-free survival (RFS) and overall survival (OS) in patients with AML who have achieved first complete remission (CR) after intensive chemotherapy, and is currently the only agent approved as maintenance therapy in AML. The combination of azacitidine (AZA) and venetoclax (VEN) is synergistic and highly effective in AML. To further improve outcomes in the post-remission setting, we studied the combination of low-dose IV/SQ AZA plus VEN as maintenance therapy in AML.
Methods: This phase II study enrolled patients with AML ≥ 18 years, not immediately eligible for SCT, and who had achieved a first CR/CRi (regardless of measurable residual disease [MRD] status) following at least 2 cycles of intensive chemotherapy (defined as intermediate or higher dose cytarabine; cohort 1) or low-intensity therapy (defined as hypomethylating agent or low-dose cytarabine-based; cohort 2). Patients in CR2 or beyond were also eligible if positive for MRD. Patients were treated with AZA 50 mg/m2 IV/SQ on days 1-5 plus VEN 400 mg PO on days 1-14, every 28 days for up to 24 cycles. VEN duration could be reduced to 7 days in patients at high risk for cytopenias. VEN dosing was adjusted for concomitant azole antifungal use. The primary outcome was RFS (defined as enrollment to relapse or death, whichever occurred first). Secondary outcomes included OS, MRD clearance rates, and safety/toxicity. Patients becoming eligible for SCT could be taken off protocol to undergo the procedure and were censored at the time of SCT. This study was registered on ClinicalTrials.gov (NCT04062266).
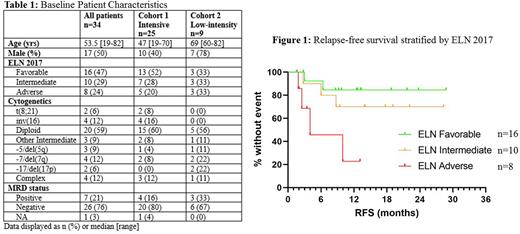
Results: As of July 14th, 2022, 34 patients have been enrolled (25 in cohort 1, 9 in cohort 2). The median follow-up time is 13.3 months (IQR 8.6-21.6). The baseline patient characteristics are shown in table 1. Nineteen (76%) patients had been previously exposed to VEN as part of their induction regimen. During cycle 1, 21 (62%) patients received 7 days of VEN and 13 (38%) received 14 days of VEN. The median number of cycles given is 9.5 (range 1-24). The median RFS is not reached (NR) in cohort 1 (70% at 12 months) and NR in cohort 2 (58% at 12 months). The median OS is NR in cohort 1 (95% at 12 months) and NR in cohort 2 (63% at 12 months). When stratified by ELN 2017, median RFS is NR (85% at 12 months), NR (70% at 12 months), and 4 months (23% at 12 months) for ELN favorable, intermediate, and adverse, respectively (figure 1). Eight patients have gone off protocol to receive SCT. There was no significant effect of prior venetoclax exposure on RFS or OS. Of the 7 MRD-positive patients at enrollment, 2 (29%) converted to MRD-negative while on maintenance therapy. The MRD-positive patients in our study had a high incidence of adverse prognostic factors (5/7 ELN adverse, 3/7 complex karyotypes). Of these MRD-positive patients, 3 went off study to receive SCT and remain in remission. The 4 remaining MRD-positive patients have relapsed after 1.9, 2.5, 4.0, and 6.0 months. The most common grade 3/4 adverse events were thrombocytopenia (21%), infections (21%), neutropenia (18%), and neutropenic fever (6%). Four patients (12%) required VEN dose reductions at cycle 2 for cytopenias. Seven (21%) patients died, all following relapse of AML or from SCT complications.
Conclusions: With over 13 months of follow up, this is the first experience demonstrating the tolerability and feasibility of low-dose AZA plus VEN as maintenance therapy in AML. RFS and OS are encouraging, especially in the non-adverse risk ELN categories (favorable or intermediate). Further studies are needed to improve maintenance strategies in patients with ELN adverse or MRD-positive disease.
Disclosures
Kantarjian:Pfizer: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Jazz Pharmaceuticals: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Honoraria. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Bose:Promedior: Research Funding; Sierra Oncology (now GSK): Consultancy; Pharma Essentia: Honoraria; Blueprint Medicines Corporation: Honoraria, Research Funding; Karyopharm: Consultancy; BMS: Consultancy, Research Funding; AbbVie: Consultancy; Novartis: Honoraria; NS Pharma: Research Funding; Astellas: Research Funding; Disc Medicine: Research Funding; Telios: Research Funding; Cogent: Honoraria, Research Funding; Kartos: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; Ionis: Research Funding; Pfizer: Research Funding; CTI BioPharma: Honoraria, Research Funding; Incyte: Honoraria, Research Funding. Jabbour:Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding. Alvarado:Sun Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; BerGenBio: Research Funding; FibroGen: Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Takahashi:Agios: Consultancy; Ostuka Pharmaceuticals: Honoraria; GSK: Consultancy; Celgene/BMS: Consultancy; Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy; Mission Bio: Honoraria; Illumina: Honoraria. Short:Amgen: Consultancy, Honoraria; Stemline Therapeutics: Research Funding; Pfizer: Consultancy; Novartis: Consultancy; AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Research Funding; Astellas: Research Funding. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Jain:MEI Pharma: Honoraria; Fate Therapeutics: Research Funding; ADC Therapeutics: Research Funding; Loxo Oncology: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pfizer: Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Cellectis: Honoraria, Research Funding; Beigene: Honoraria; Takeda: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Dialectic Therapeutics: Research Funding; Incyte Corporation: Research Funding; Medisix: Research Funding; TG Therapeutics: Honoraria; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Ipsen: Honoraria; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Newave: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Mingsight: Research Funding; Servier Pharmaceuticals LLC: Research Funding; Novalgen: Research Funding; Aprea Therapeutics: Research Funding; TransThera Sciences: Research Funding; Cellectis: Honoraria, Research Funding; CareDx: Honoraria. DiNardo:Foghorn: Honoraria, Research Funding; Novartis: Honoraria; Takeda: Honoraria; AbbVie: Consultancy, Research Funding; LOXO: Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; Bluebird Bio: Honoraria; Forma: Research Funding; Cleave: Research Funding; Astex: Research Funding; Astellas: Honoraria; ImmuneOnc: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Gilead: Honoraria. Burger:Pharmacyclics LLC: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Janssen: Consultancy, Speakers Bureau; Gilead: Consultancy; TG Therapeutics: Consultancy; Novartis: Consultancy. Ferrajoli:Beigene: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Novartis: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; Aprea: Honoraria; Genentech: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Acceleron Pharma: Consultancy. Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ravandi:AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Syos: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea Fusion, Inc.: Research Funding. Kadia:Genentech: Consultancy, Research Funding; Servier: Consultancy; Iterion: Research Funding; AstraZeneca: Research Funding; Genfleet: Research Funding; Amgen: Research Funding; Ascentage: Research Funding; cellenkos: Research Funding; BMS: Consultancy, Research Funding; Glycomimetics: Research Funding; PinotBio: Consultancy; Regeneron: Research Funding; Astex: Honoraria; Abbvie: Consultancy, Research Funding; Astellas: Research Funding; cyclacel: Research Funding; JAZZ: Consultancy, Research Funding; Delta-Fly: Research Funding; Pfizer: Research Funding; Novartis: Consultancy; Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal